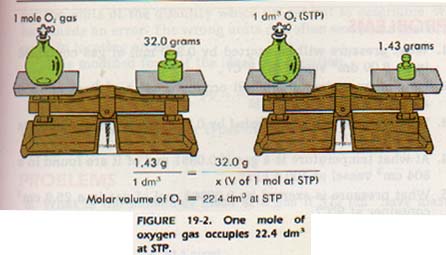
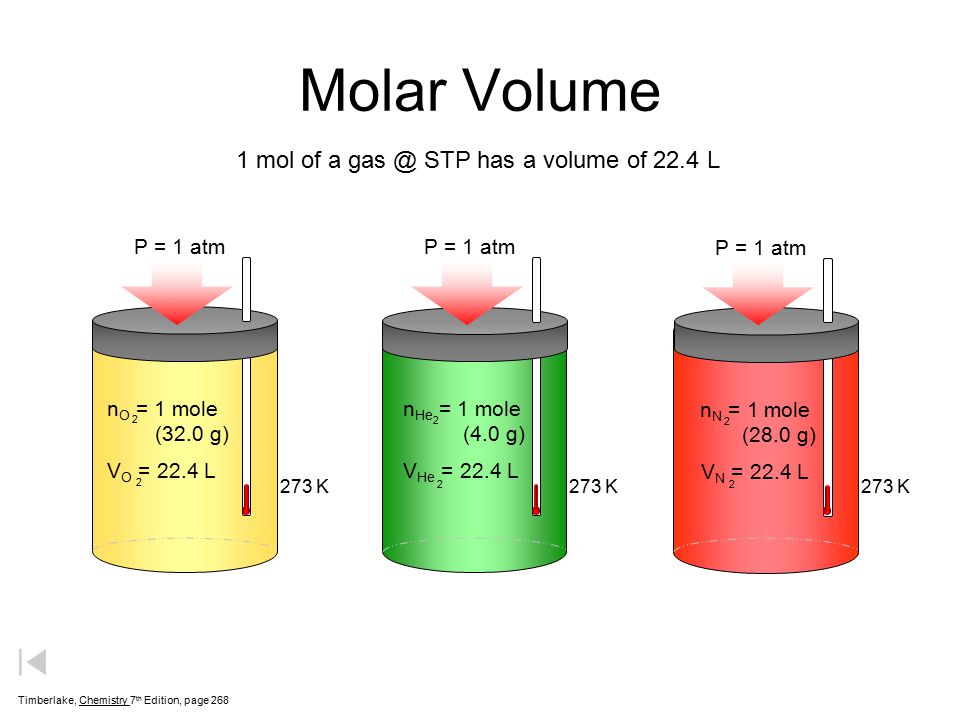
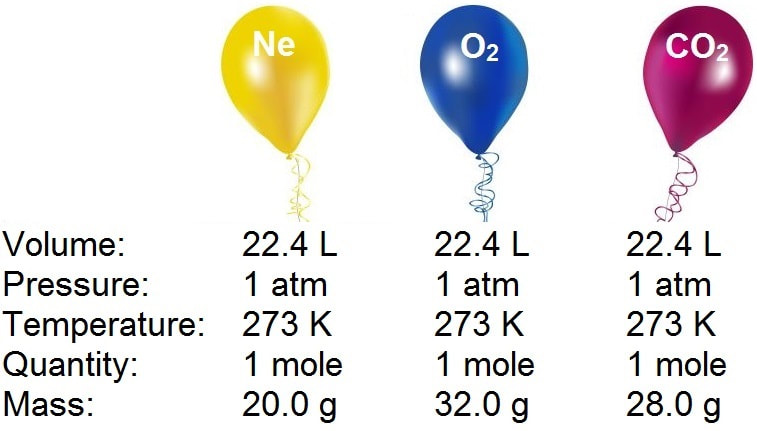
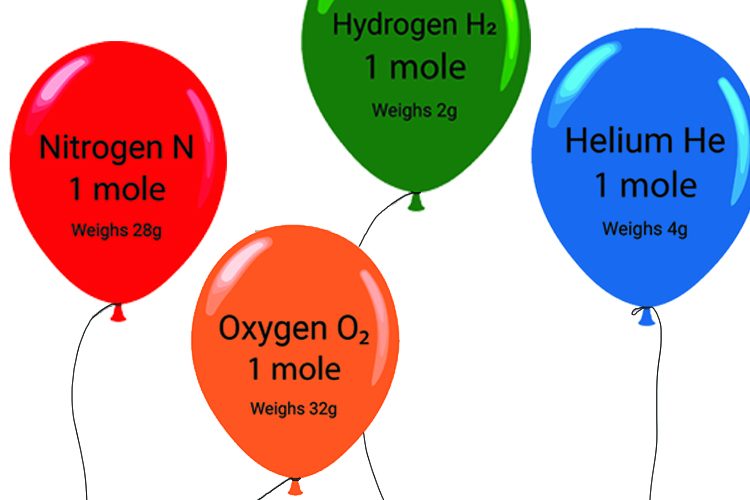
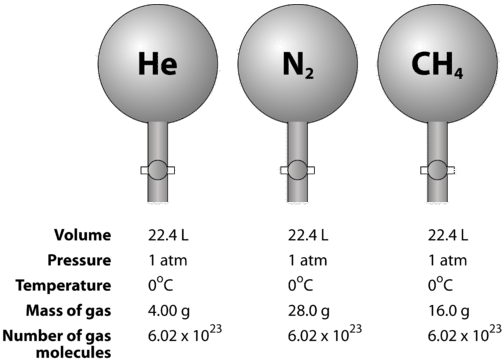
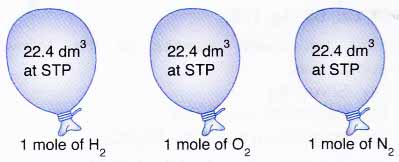
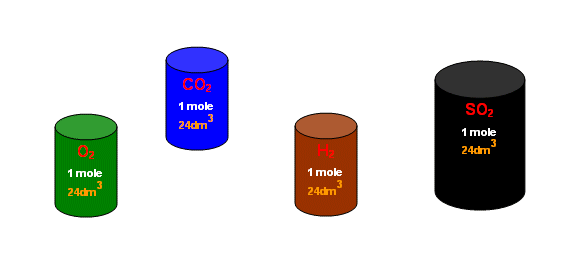
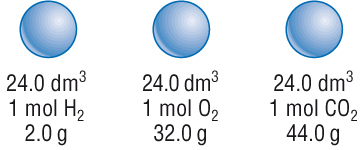
20. What is the exact volume of gas a NTP and STP and what is the temperature and pressure at both comditions

47. 38. The weight of 1 mole of a gas of density 0.1784 g L-' NTP is 21 22 10.1784 g (2) 19 (3) 4g (4) Can not be calculate

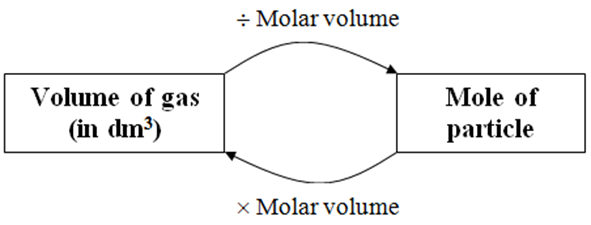
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

gas laws - Why 1 mole of H2 occupied the same volume occupied by 1 mole of O2? - Chemistry Stack Exchange
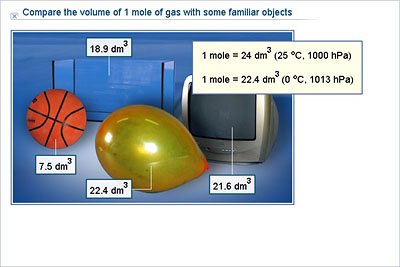
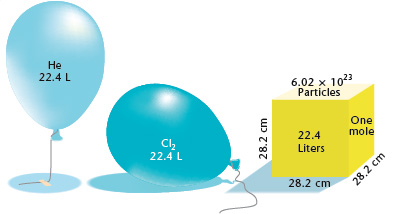
Chemistry - Lower Secondary - YDP - Illustration - Compare the volume of 1 mole of gas with some familiar objects

Molar volume is the volume occupied by 1 mole of any (Ideal) gas at standard temperature and pre... - YouTube

SOLVED: The volume of 1 mole of a gas is 0.022414 m^3. Will a blast furnace gas containing 2.2 kg CO, 1.46 kg CO2, and 4.3 kg N2 oxidize Fe at 700°C?