SOLVED: In the reaction of 2-methylbut-1-ene with HBr, there are two possible carbocation intermediates; one is classified as tertiary (3°) and the other as primary (1°). The structures of the starting alkene

reaction of ALKENES with bromine water but-2-ene but-1-ene cyclohexene hex-1 -ene hex-2-ene ethene propene skeletal formulae ionic electrophilic addition mechanism advanced A level organic chemistry revision notes doc brown

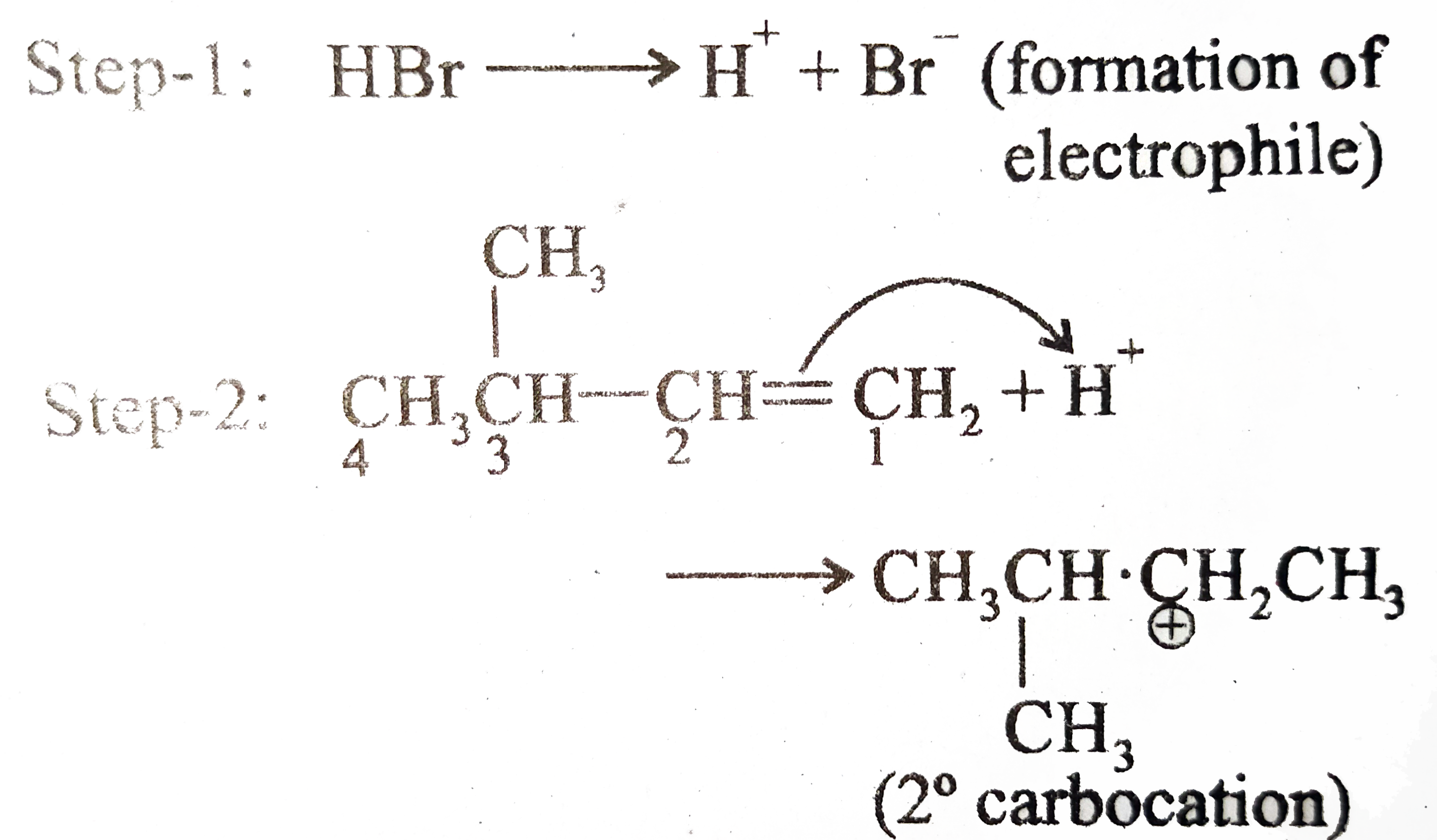
The reaction of 1-butene with HBr in the absence of peroxides yields 2-bromobutane. The mechanism for the reaction involves: A. Isomerization of the 2-bromobutane produced initially B. Attack on the alkene by

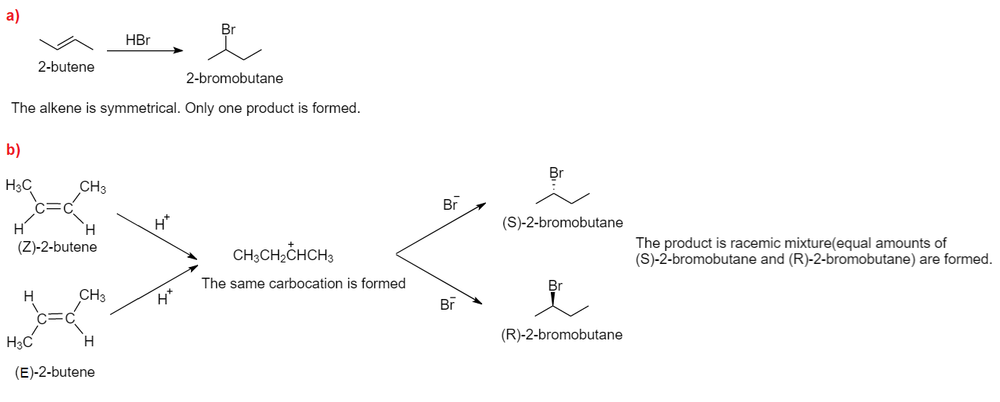
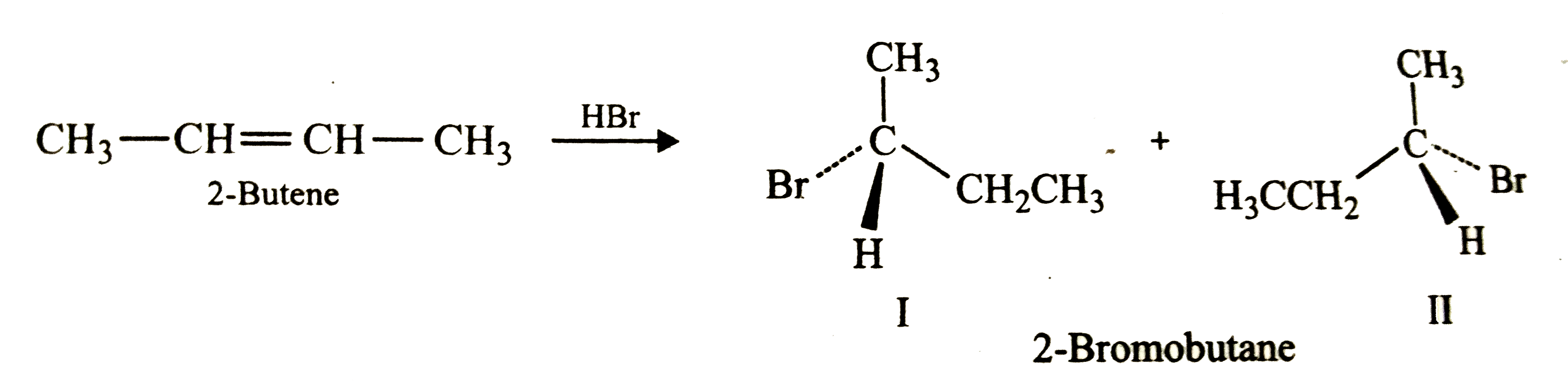
ReasonAddition of HBr on 2-butene follows Markovnikov's rule.AssertionAddition of HBr on 2-butene gives two isomeric products.

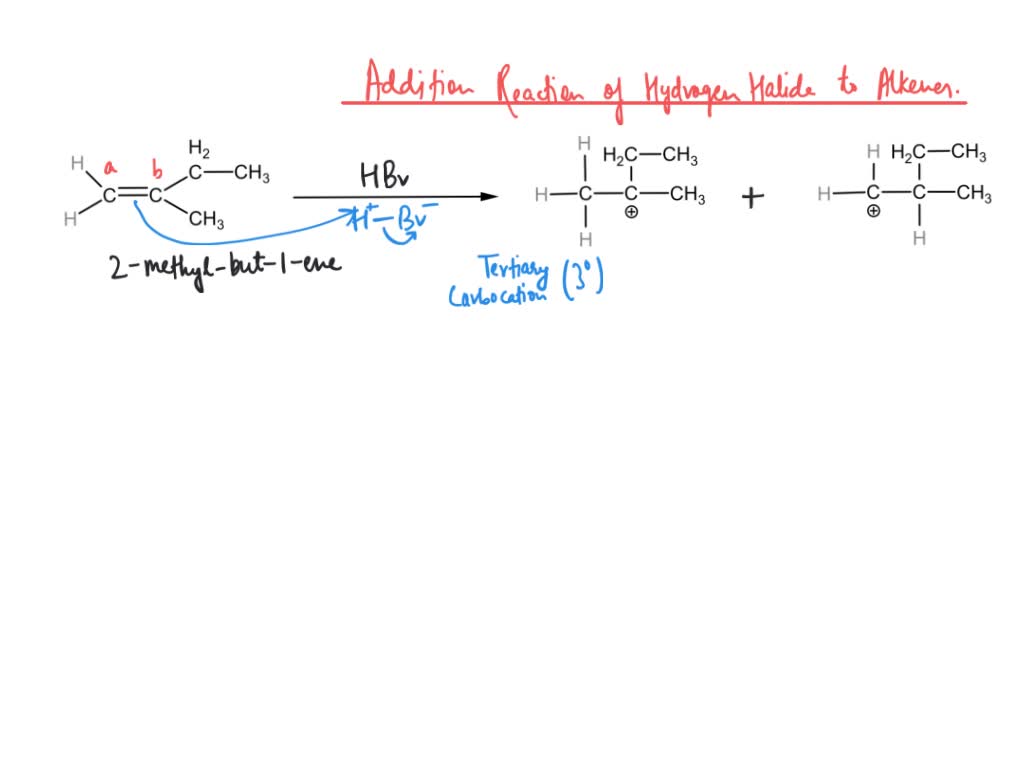
When 2-methyl-1-butene is treated with HBr, only one product forms: 2-bromo-2-methylbutane. Why? A. The reactants have read the published papers of Vladimir Markovnikov, and dutifully obey his rule. B. 2-bromo-2-methylbutane is the

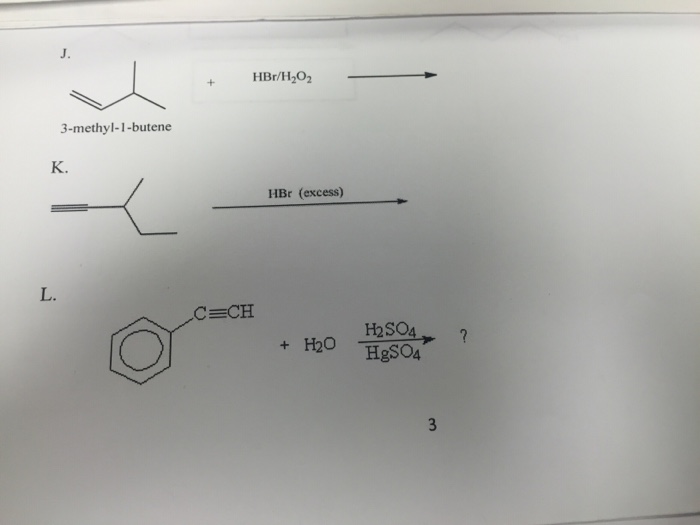
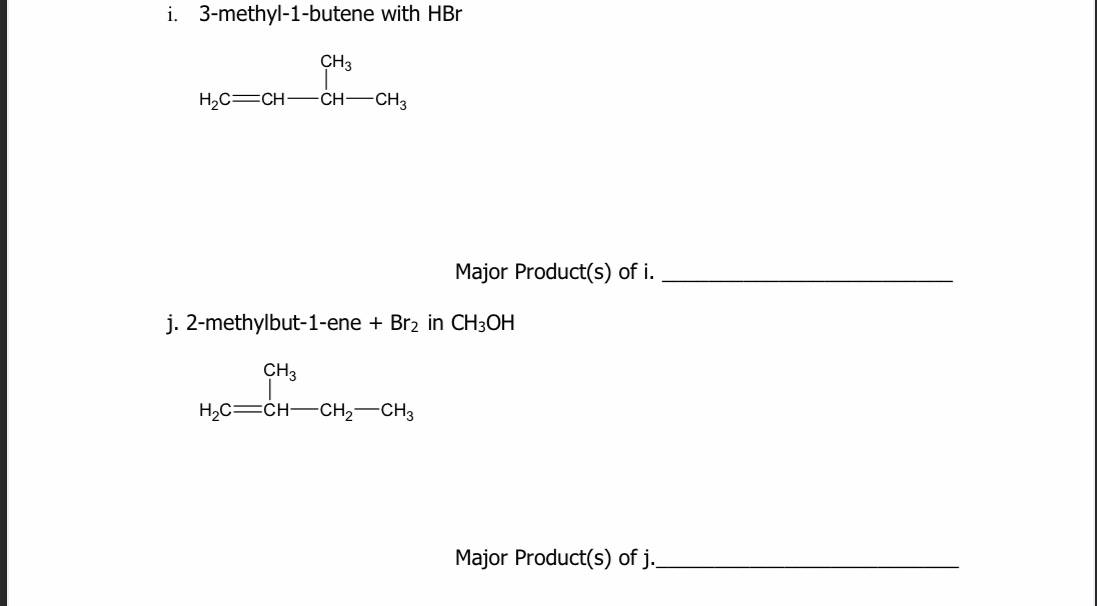
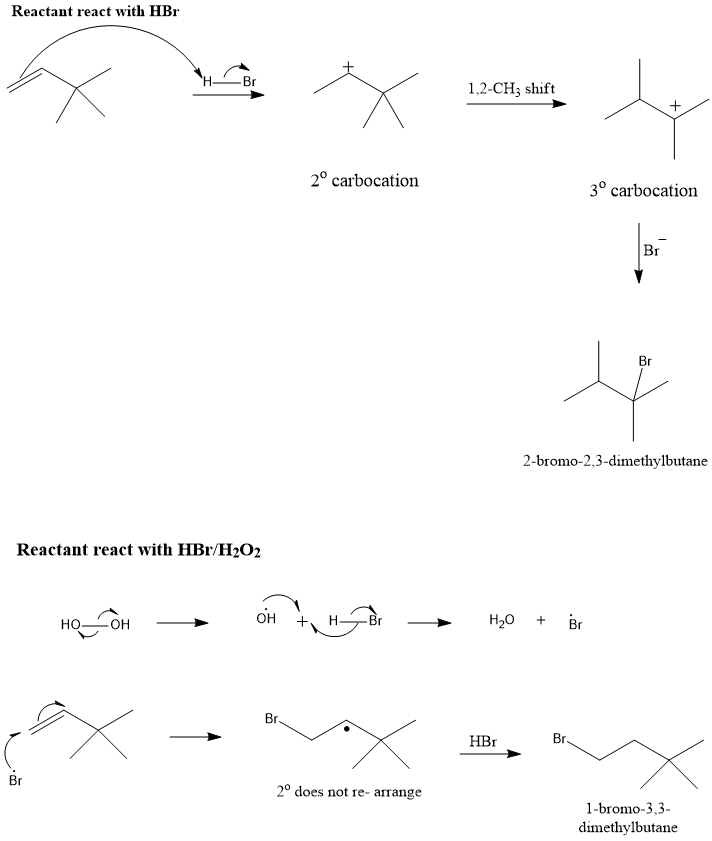
Consider the addition of HBr to 3,3-dimethyl-1-butene, what is the mechanistic explanation the formation of observed product?

The addition of HBr to 1-butene gives a mixture of products (I),(II) and (III):The mixture consists of :

Predict the major and minor products of 2-methyl-2-butene with HBr as an electrophilic addition reaction. Include the intermediate reactions. | Homework.Study.com

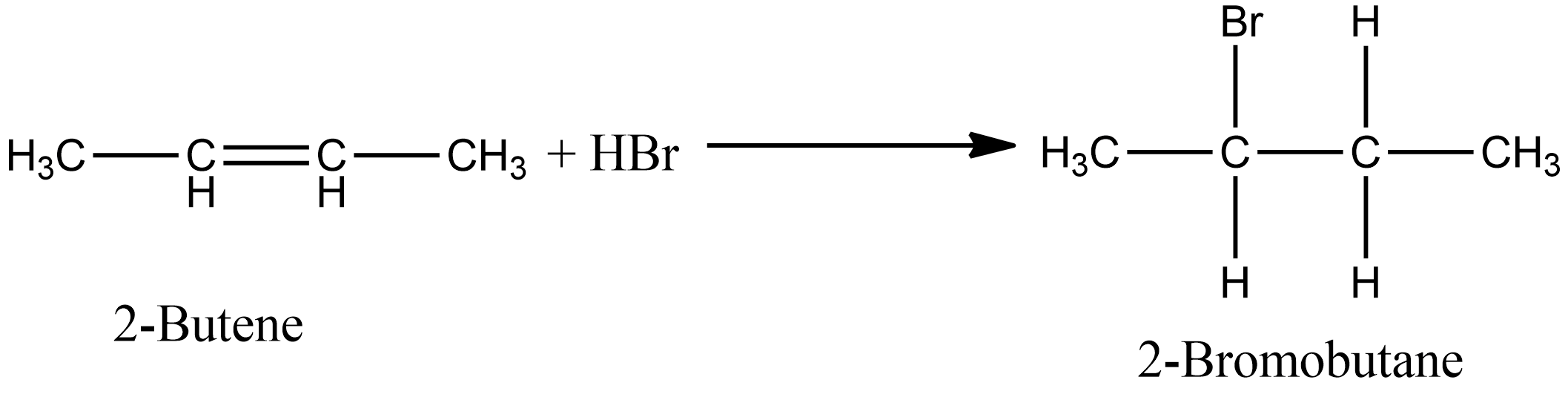
The addition of HBr to 2-butene produces: a. 1-bromobutane b. 2-bromobutane c. 1,2-dibromobutane d. 2,3-dibromobutane e. no reaction | Homework.Study.com
Statement 1: 1-Butene on reaction with HBr in the presence of a peroxide produces 1 -bromo-butane. Statement 2: It involves the free radical mechanism.
Statement I : 1-butene on reaction with HBr in the presence of a peroxide produces 1 -bromobutane. - Sarthaks eConnect | Largest Online Education Community

reaction of ALKENES with bromine water but-2-ene but-1-ene cyclohexene hex-1 -ene hex-2-ene ethene propene skeletal formulae ionic electrophilic addition mechanism advanced A level organic chemistry revision notes doc brown